
Maintenance-free, durable products
Certified quality from a single source
40 Jahre Know-How
Made in Germany

Start | Industries | Pharmaceutical and medical technology

For the production, storage and packaging of pharmaceutical products, sterile ultra-pure water is needed as well as germ-free air.
Pure water from the collecting tank is needed by production of non-steril products. It also used for cleaning applications without any microbiologic and chemical substances.
Read more about ultrapure water for pharma applications.

The water quality is requried for preparation of medical products, which has to be controlled. The HPW is produced by osmis methods and ultrafiltration.
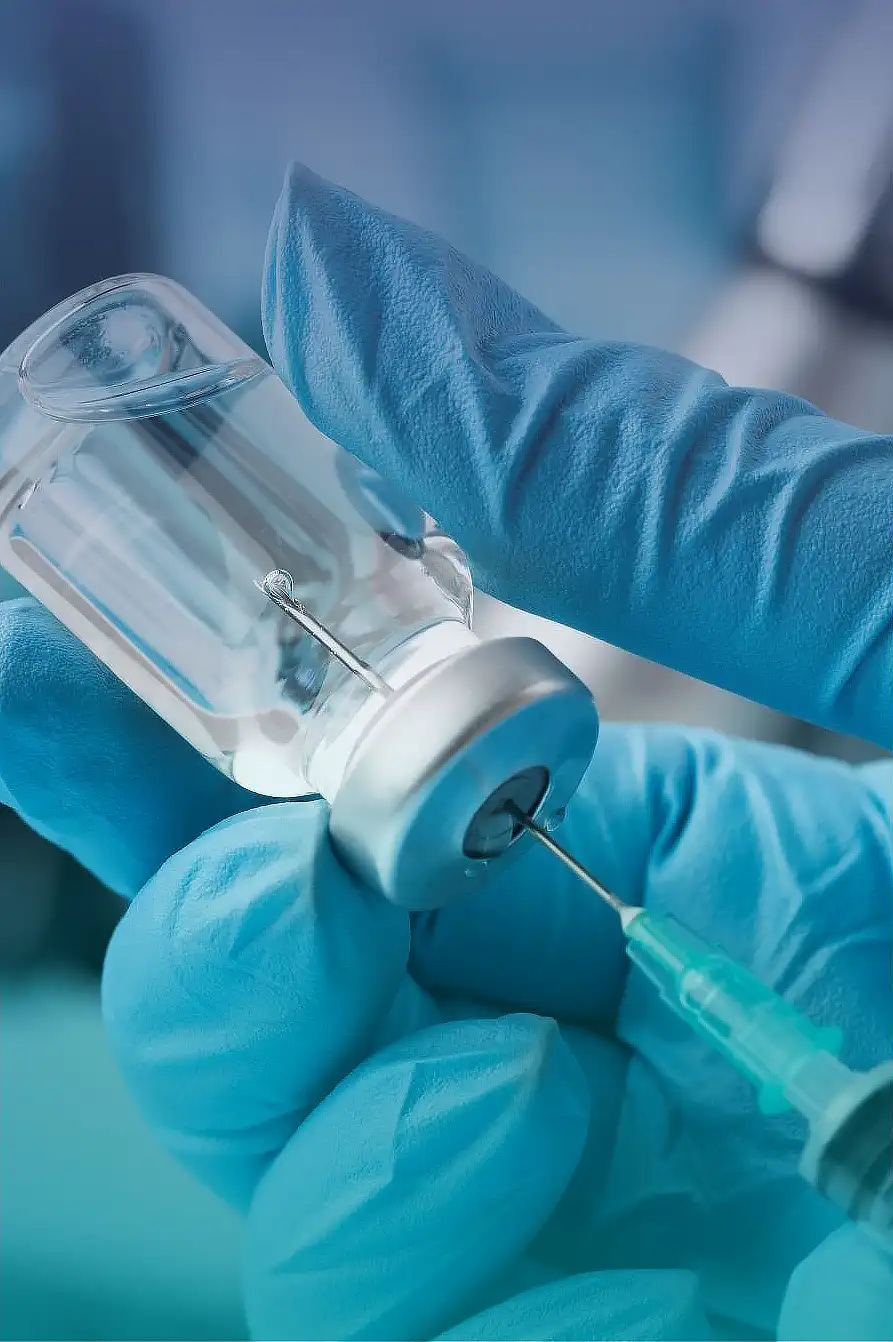
Injection water is made by the highest cleaning phase. The utrapure injection water is used for dissolving substances. The quality is concretly defined.
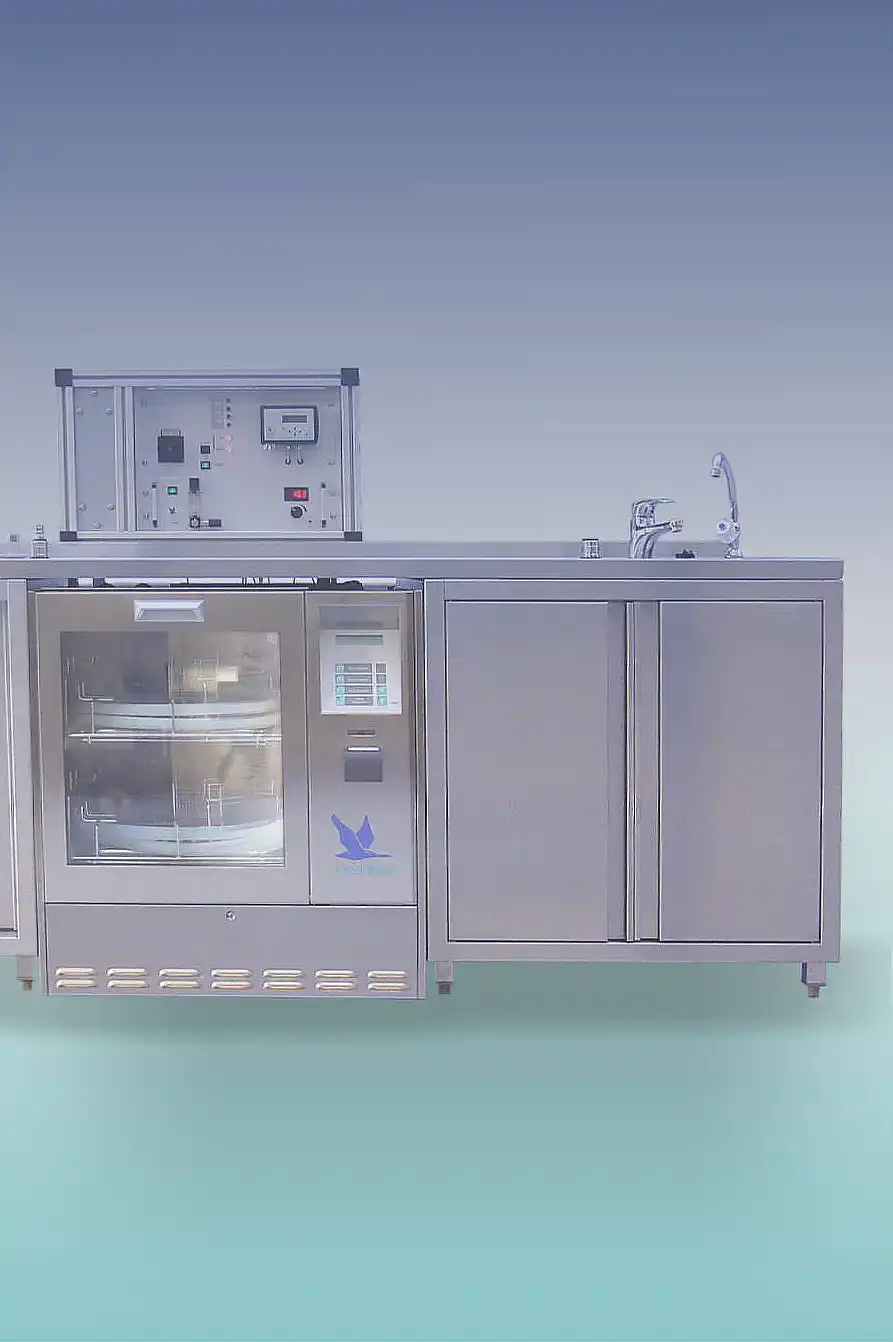
For sterilization and decontamination of equipment, tools, rooms, clothing and footwear, certified equipment is available.
More information on sterilized water for medical applications.
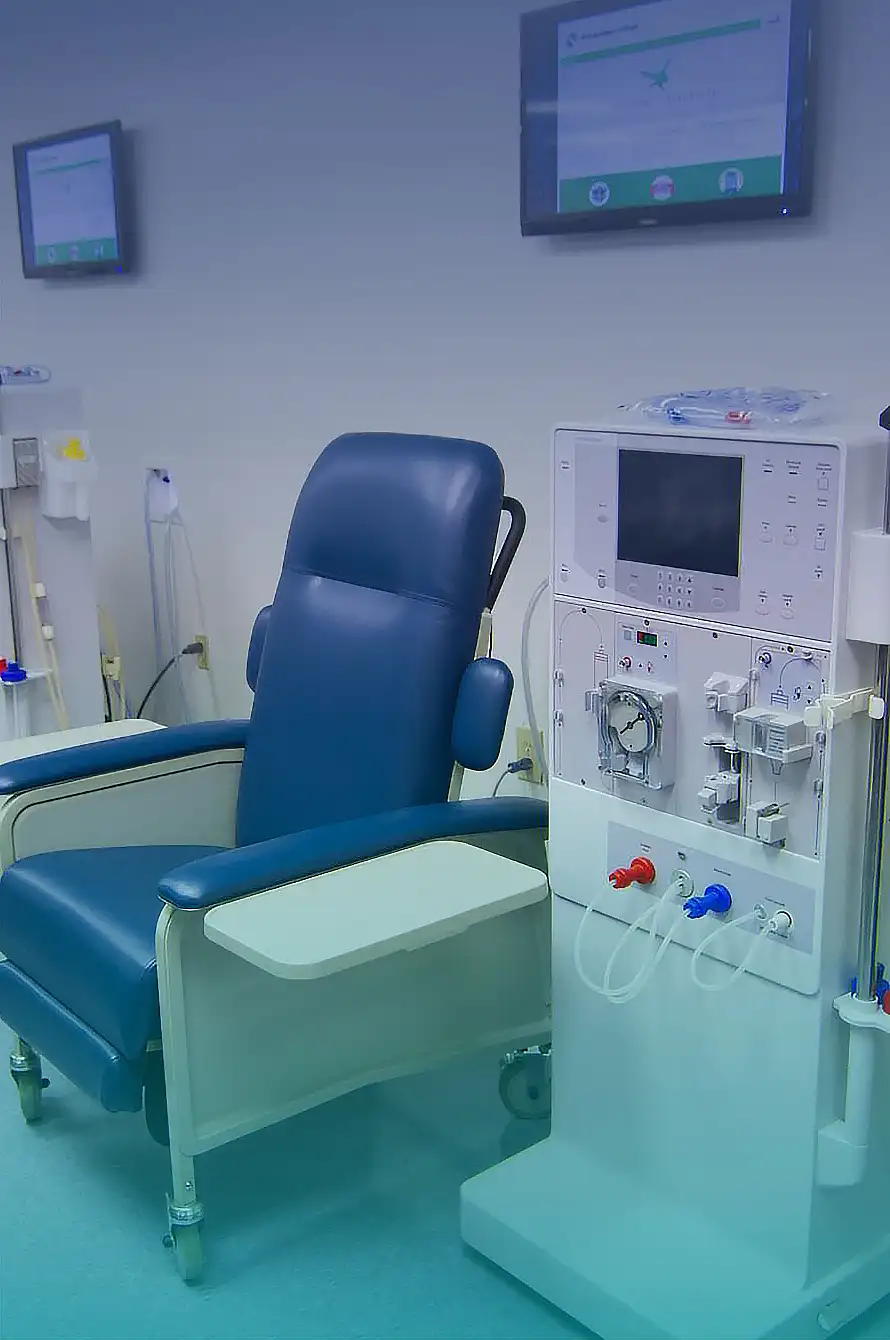
The healing and anti-inflammatory effect of ozone is well-known. Learn more about principles and applications of hemodialysis proceedings.
The European Commission of Drug and Medicine has made two classifications for water quality.
“Water for injection purpose” and “water for industrial purpose“.
The germ number for “water for industrial purpose” should be below 10 cfu/100ml, probe and conductivity below 5 µS/cm. In order to receive proper validation requirements by the pharmaceutical industry can individually differ.
After deionisation ultra filtration is a possibility to receive bacteria and pyrogen-free (BGF) water. In order to avoid any bacteria growth or bio-slime inside of the distribution a loop ozone has to be used continuously. Bio film can be controlled by coupons installed in the circuit.
For safeguarding biological contamination in the DIW loop it is important to have dissolved ozone available, wherever and whenever. Discover our ultra-pure treatments for your specific application.

